Average Kinetic Energy Per Molecule of a Gas
The average kinetic energy of a collection of gas particles is directly proportional to absolute temperature only. Calculate the temperature T of a sample of gas when the average translational kinetic energy of a molecule in the sample is 839 x 10-21.
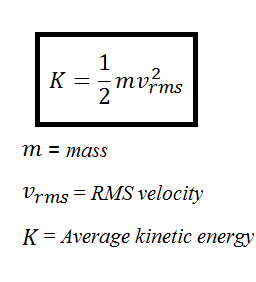
Average Kinetic Energy Temperature Of A System Video Lesson Transcript Study Com
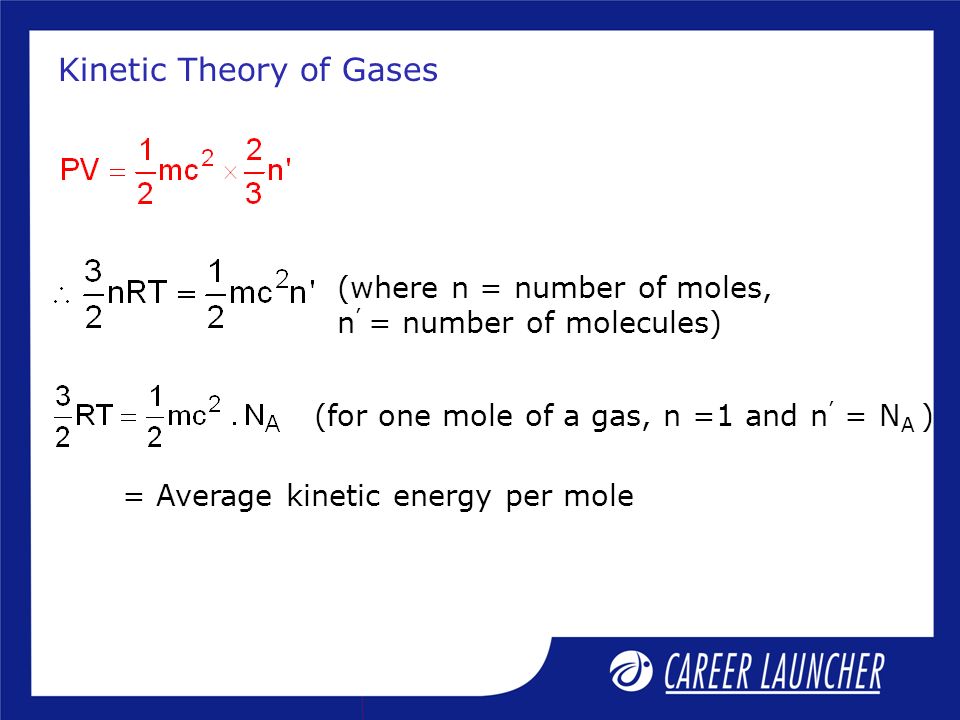
We know that PVnRT 9 Thus equating equation 8 and 9 we get-.

. T 4053 K What is the total translational kinetic energy Ktrans of all the molecules of this sample when it contains 215 moles of gas. 1 7 1 0 2 1 Jmolecule. The kinetic energy per molecule per degree of freedom is 12kt.
Where m is the mass of the gas molecule. Correct option is B The average kinetic energy of an ideal gas per molecule in SI units at 25 C will be 61710 21 J. The quantity m v 1 2 m v 2 represents the average translational kinetic energy of an ideal gas molecule.
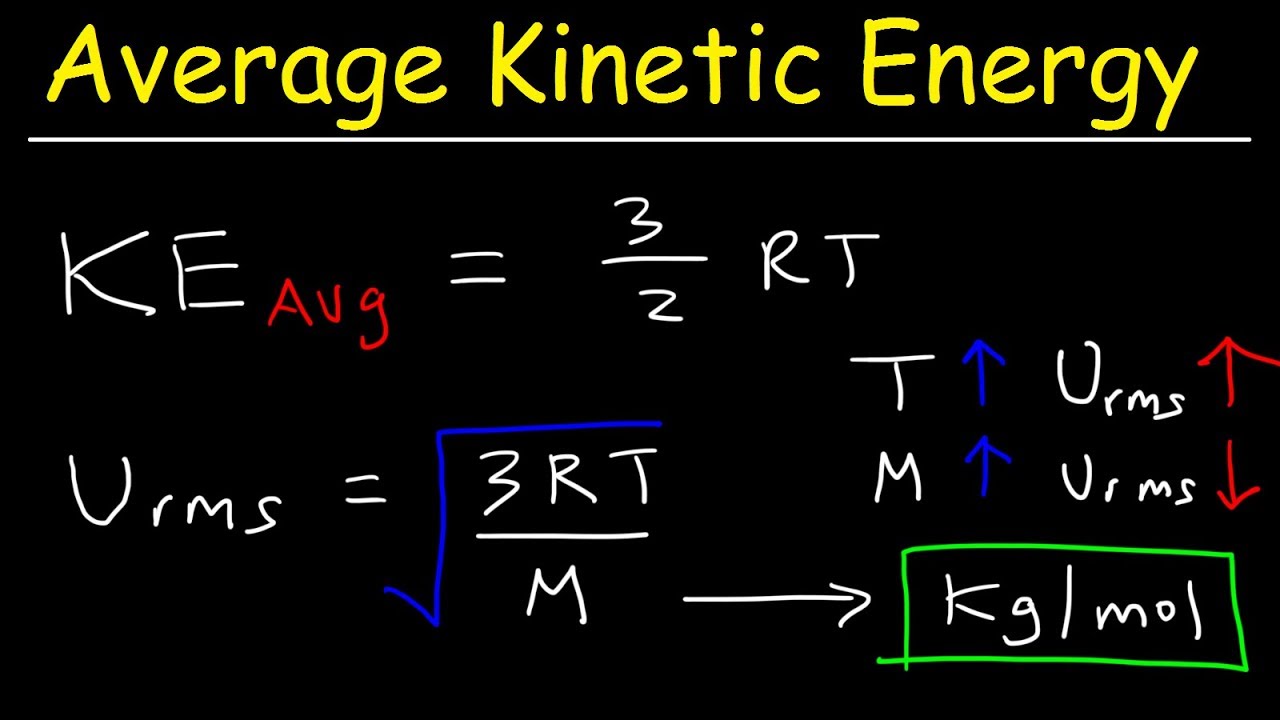
Hope it helpful to you. Thus the average kinetic energy of translation of a gas molecule ½mv² rms k depends on its temperature and its independent of pressure volume or nature of. Average kinetic energy 3 2 RT per mole 3 2 KT per molecule 15 KT per molecule.
The average translational energy and the rms. A v e r a g e K. For every molecule there are three rotational degree of freedom.
There is also no energy associated with bonds between atoms in molecules because there are no bonds in a monatomic gas. 617 10 21 J. 100 2 ratings average KE per molecule 32kT.
This is the best answer based on feedback and ratings. 3 2 8313 J m o l K 6023 10 23 m o l 298 K A v e r a g e K. The following is the deduction of kinetic theory in terms of pressure.
What is the Average Kinetic Energy of a Gas Molecule. 2 E 3 2 Nk B T. A monoatomic gas has 3 degrees of freedom one each along for each axis translation motion.
The correct option is C. The kinetic energy per molecule for a monoatomic gas is 32kt option 2 is correct. The kinetic energy is measured in Joules J and the temperature is measured in Kelvin K.
If both the statement are TRUE and STATEMENT-2 is the correct explanation of STATEMENT-1 B. 0 2 3 1 0 2 3 moleculesmol 8313 JmolK 298 K. Kinetic Energy of Gas Formula.
The average kinetic energy per molecule of helium gas at temperature T is E and the molar gas constant is R. Average kinetic energy 3 2 k T. The average KE of a gas particle has 12kT per energy mode k is Boltzmanns constant and T is its absolute temperature in Kelvin.
K average kinetic energy per molecule of gas J. The formula for the kinetic energy of a gas defines the average kinetic energy per molecule. The kinetic energy of the translational motion of an ideal gas depends on its temperature.
Here k is Boltzmann constant and T is absolute temperature. Then Avogadros number is equal to RT 3RT 2RT 2E 3 5 433RI 3RT 2E Open in App. For T 37 273 K we are easily in the required high-temperature limit.
Average kinetic energy K E 2 3 k T 2 3 N R T K E 2 3 6. Therefore the total energy of the gas is E N m v 1 2 m v 2 Substituting in equation 1 PV 2 3 E. Boltzmanns constant is k 138.
The average kinetic energy of the molecules in a gas sample depends only on the temperature 𝑇T. P m n v 2 3. The average kinetic energy of an ideal gas per molecule in S I unit at 2 5 o C will be 6.
What is the average kinetic energy per molecule of an ideal gas at a temperature of 300 K. Thus E 15 310 138 10-23 J implies E 6417 10-23 J Or E 00401 eV. The average translational energy of a molecule is given by the equipartition theorem as E 3kT2 where k is the Boltzmann constant and T is the absolute temperature.
Speed of molecules in a sample of oxygen gas at 300 K are 621 10-21 J and 484 ms respectively. However given the same kinetic energies a lighter molecule will move faster than a heavier molecule as shown in the equation for rms speed. The average kinetic energy of an ideal gas per molecule in SI units at 25 C is 617 10 21 J.
EqE frac 3 2Nk_ bT eq where E is the average kinetic energy of the gas T is the. The kinetic energy of the translational motion of an ideal gas depends on its temperature. The kinetic energy is measured in Joules J and the temperature is measured in Kelvin K.
K 136 10 23 J K. The formula for the kinetic energy of a gas defines the average kinetic energy per molecule. Where ½ mv² rms Average kinetic energy of translation K of a gas molecule.
Use the following formula for the average kinetic energy of an ideal gas per molecule. From ideal gas equation PV Nk B T 2 3 E. The average translational kinetic energy per molecule of the gas per degree of freedom is 12 KT.
See the answer See the answer done loading. N is the number of. Therefore the option B is correct.
View the full answer. Where 𝑅8314 J molKR8314 J molK and ℳℳ is molar mass in kilograms per mole. The average kinetic energy of a gas molecule can be determined by knowing.
The result above says that the average translational kinetic energy of a molecule in an ideal gas is 32 kT. We know that the average kinetic energy of an ideal gas per molecule is given by the expression. So for a monatomic particle it has an av KE of 32kT 12 kT for each translational mode of which it has three for the three independent directions in which it can travel.

The Average Kinetic Energy Of A Molecule Of A Gas At Absolute Temperature T Is Proportional To Youtube

A What Is The Average Translational Kinetic Energy Of A Molecule Of An Ideal Gas At Temperatur Youtube

Average Kinetic Energy Per Molecule Of An Ideal Gas Is Given Scholr

The Kinetic Energy Per Molecule Of A Gas At Temperature T Is

The Average Kinetic Energy Of An Ideal Gas Per Molecule In Si Unit At 25 Oc Will Be

The Average Kinetic Energy Per Molecule Equation For An Ideal Gas Ib Physics Youtube

The Average Kinetic Energy Per Molecule Equation For An Ideal Gas Ib Physics Youtube

Find The Average Translational Kinetic Energy Per Molecule If One Mole Of The Gas Is Contained In A Volume 1 23 10 3 M 3 At A Pressure 2 10 5n M 2 Avagadro S Number Is 6 02 10 23 Molecules Moles
What Would Be The Average Kinetic Energy Of A Hydrogen Molecule At 300k Quora

The Average Translational Kinetic Energy Of Air Molecules Is 0 040 Ev 1ev 1 6xx10 19 J Ca Youtube

Chemistry Of Gases 32 Of 40 Kinetic Energy Of A Gas Molecule Youtube
Chemistry Ppt Video Online Download
Average Kinetic Energy Of A Gas And Root Mean Square Velocity Practice Problems Chemistry Gas Laws Youtube
Show That Average Kinetic Energy Of Translation Per Molecule Of Gas Is Directly Proportional To The Absolute Temperature Of Gas Sarthaks Econnect Largest Online Education Community

Unit 6 Gases The Kinetic Molecular Theory Ppt Video Online Download

Average Kinetic Energy Of A Gas Molecule Is

Average Kinetic Energy Temperature Of A System Video Lesson Transcript Study Com

Physical Chemistry Kinetic Energy Of An Ideal Gas Chemistry Stack Exchange

Average Kinetic Energy Temperature Of A System Video Lesson Transcript Study Com
Comments
Post a Comment